OCR Physics Combined Sciences

Radioactivity
P4.3-1 Atomic structure
There are particles in an atom: electrons, protons, and neutrons. The two last ones are in the nucleus and are called nucleon.
Proton is positive, electron is negative, and neutron is neutral.
Number of protons in an atom determines what chemical element it is.
Isotopes of an element have different number of neutrons.
Element symbols:

Number of neutrons in an atom = A- Z
In a neutral atom the number of electrons and protons are equal.
Ion: when an atom gains or loses electrons.
If it gains it is negatively charged (or negative ion), and if loses it is positive.
Note: protons are fixed in the nucleus, if they could move the element would be change!
Bohr showed electrons are arranged in distinct energy levels around the nucleus. Electrons can move up and down the energy levels thanks to Electromagnetic (EM) radiation:


The difference between energy levels of electrons determines the wavelength of the EM wave.
P4.3-2 Radioactive Decay
Radioisotope: an unstable atom that undergoes radioactive decay to become more stable.
Radioactivity is random, meaning we don’t know when a particular nucleus will decay. Radioactivity does not depend on temperature.
Activity: number of nuclei (plural of nucleus) that decay per second – unit: Becquerel (Bq) or count per second.
There are four decay radiations: alpha, beta, neutron, and gamma
Alpha Decay: alpha is made of two protons and two neutrons (helium nucleus). When an alpha particle is released from the nucleus of an atom the atomic number decreases by 2, but the mass number decreases by 4. The decay product is a different element.
Beta Decay: a neutron in the nucleus changes to a proton and an electron, the electron is ejected from the nucleus becomes the beta radiation. After beta decay atomic number increases by 1; the mass number does not change! Result is a new element.
Gamma Decay: gamma is a high energy EM wave, without any mass, or charge (gamma is not a particle like electrons or protons). They are produced when an electron falls into a lower energy level in the atom. Mass number and atomic number is unchanged.
P4.3-2-a Radioactive Decay Equations
For these equations, the total of mass number, atomic number and charge should be the same on both sides of the equation (equation should be balanced). The atomic number can be used as charge as well because it shows the number of protons.
Alpha decay equation:

Beta decay equation

We normally don’t write an equation for gamma radiation, as it just happens when an electron falls to a lower energy level, so there are no changes in mass or atomic number of the element.
P4.3-2-b Background radiation
It is ionising radiation that around us almost everywhere!
It comes from rocks (like granite used in kitchen tops), cosmic rays coming from the space, nuclear weapon testing, and some from medical applications (like X-Ray imaging for bones). Even we all are a little radioactive because of the radioisotopes in our body.
Background radiation from our activities are from different sources such as: waste from nuclear power plants, army, and hospitals.
The unit for radiation dose is Sieverts (Sv).
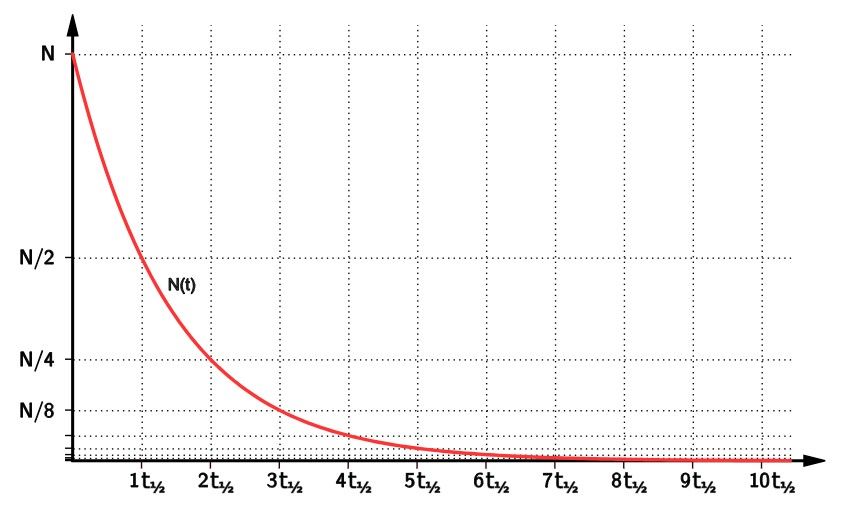
P4.3-2-c Half-life
It is the time it takes for half of the radioactive nuclei (plural of nucleus) to decay.
Or
The time for the activity of the radioactive material to half!
Or the time for count rate to half!
Graph gets very close to the x-axis (time) but will never touch it!
Smoke alarms use a radio isotope with a long half-life (americium-241), because otherwise the detector would not be reliable and needed replacing often.
Some radioisotopes are used inside human body to trace biological process or cancerous cells. These should have very short half-life to reduce the danger to the patient from the ionising radiation.
Higher only:
Calculating Half-life:
- The background radiation should be deducted from any reading of the Geiger-Muller Tube
- Draw the graph of half-life: activity vs. time
- By finding the time it takes the activity to half, show this time on several points of the graph, e.g. from 100 counts/minute to 50; and from 80 to 40 etc. these values will be different due to random nature of radioactive decay;
- Calculate the average of the values found in the previous step.
Example 1:
Half-life of Iodine-131 is 8 days. A sample of iodine-131 has an activity of 1600 Bq.
- Calculate the count rate after 40 days;
- Calculate the net decline of activity.
Count rate | 1600 | 800 | 400 | 200 | 100 |
Days | now | 8 | 16 | 32 | 40 |
Net decline is ratio of count rate now over the original: in this case 40/1600 = 1/40 = 0.025.
Example 2: (higher only)
Determine the half-life of carbon-14 from the graph below:

Solution:

From the vertical axis we find when the amount of active carbon-14 nuclei becomes half, and then from the point on the graph we find the time it takes for that to happen.
In this case it is about 5500 years!
If the vertical axis shows the activity in Bq (or another unit such as counts per minute) same approach is taken.
P4.3-2-d Penetration of radiations

Alpha:
- Least penetrative, can be stopped by sheet of paper;
- Used in smoke detectors as it can be stopped by few centimetres of air;
- Least dangerous outside our body; most dangerous inside the body;
- Most ionising because has the largest mass and biggest charge.
Beta:
- Can be stopped by sheet of metal (3 mm of aluminium);
- Medium ionising, and penetration.
Gamma:
- Most penetrative, can be stopped by several metres of concrete or several centimetres of lead;
- Least ionising.
P4.3.2-e Contamination and irradiation
Contamination means the undesirable presence of radioactive material.
- Radioactive material can enter human body, through eating, breathing, or entering via a wound, or can be absorbed via skin.
- Contamination with these material can cause mutation if genes, and cancer.
The level of contamination depends on:
- penetrative level of radiation;
- level of ionisation of it
This is why alpha is most dangerous when inside the body! It is the most ionising but least penetrative. It cannot much damage when it is outside the body.
Medical tracers are radioactive isotopes that injected or eaten by a patient, to monitor the function of their internal organs, or check if their veins are blocked. The radiation should be penetrative enough to be detectable by scanner outside the patient’s body, and it should have a short half-life, so that it does not expose the patient and people around to too much radiation! It is advised that these people stay away from babies and pregnant women for several days after taking the radioisotope!
Irradiation is the exposure of an object to radiation.
Sources of radiation: food, sun, earth, building material, nuclear waste from hospitals, power plans, or army.
Gamma rays is used to sterilise food by super markets or hospitals.
Irradiated DNA of a cell, may cause damaged nucleus which causes mutation and can cause cancer, or the cell may die straight away. Damaged genes of people exposed to radiation may continue to mutate in future generations and cause birth defects.
It is important that effects of radiation are published in scientific journals so that other scientists are aware of the research. If a scientific research is peer reviewed it means its results can be repeated by other researchers.
Revise and Get Paid!
If you like taking summary notes of lessons and solving past papers, see the Join Us page!